
Apart from these seven rows, there are two rows of elements placed separately at the bottom of the periodic table. (2) Each box represents the place for one element. Atomic numbers are serially indicated in the upper part of these boxes.
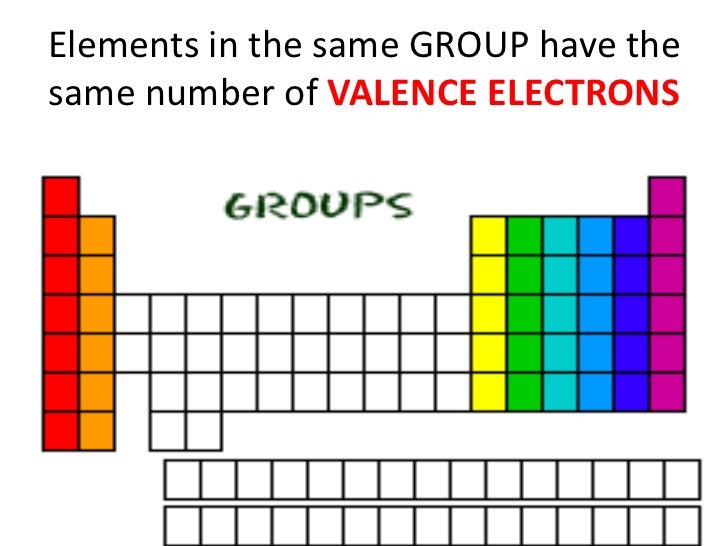
The arrangement or the periods and groups results into formation of boxes. In the modern periodic table there are seven horizontal rows called periods and eighteen vertical columns (1 to 18) called groups. (1) In the modern periodic table, the elements are arranged in the order of their increasing atomic number. The physical and chemical properties of elements are a periodic function of their atomic masses.ī. On the basis of these finding Mendeleev stated the periodic law. When the elements are arranged in the order of their increasing atomic masses, Mendeleev found that the elements with similar physical and chemical properties repeat after a definite interval. The noble gas with the smallest atomic radius.Ĭlass 10 Science Chapter 2 Periodic Classification Of Elements Question 6. The atom having the smallest atomic mass.ĭ. Write the name and symbol of the element from the description.ī. Science 1 Chapter 2 Class 10 Maharashtra Board Question 5. 6C, 3Li, 9F, 7N, 8O Which of the above elements has the highest nonmetallic character?ĩF has the highest non metallic character. 13Al, 14Si, 11Na, 12Mg, 16S Which of the above elements has the highest metallic character? 19K, 3li, 11Na, 4Be Which of these atoms has smallest atomic radius? 11Na, 15P, 17Cl, 14Si, 12Mg which of these has largest atoms?į. 4Be, 6C, 8O, 5B, 13Al Which is the most electropositive element among these?Īmong these, 13Al is the most electropositive element.Į. 7N, 6C, 8O, 5B, 13Al Which is the most electronegative element among these?Īmong these, 8O is the most electronegative element.ĭ. Which of these elements belong to the second group?Įlements belongs to the 2nd group: 4Be and 20Ca.Ĭ. 3Li, 14Si, 2He, 11Na, 15P which of these elements belong to be period 3?Įlements belong to the 3rd period: 14Si, 11Na and 15P.ī. Answer the following question with explanation.Ī. Write down the electronic configuration of the following elements from the given atomic numbers.
With which of the following elements would this element resemble? (Atomic numbers are given in the brackets)Ĭlass 10 Science 1 Chapter 2 Periodic Classification Of Elements Question 4. To which period does this element belong?ĭ. What is the atomic number of this element?Ĭ. (d) In which block of the modem periodic table are the nonmetals found?Ĭlass 10 Science Chapter 2 Periodic Classification Of Elements Notes Question 3.Īn element has its electron configuration as 2, 8, 2. which of the following elements be present in the same group as X. This compound is a solid having high melting point. (c) Molecular formula of the chloride of an element X is XCl. This means that their position in the modern periodic table is in……. (b) Alkaline earth metals have valency 2. (a) The number of electrons in the outermost shell of alkali metals is……. Periodic Classification Of Elements Class 10 Maharashtra Board Question 2.Ĭhoose the correct option and rewrite the statement: Lightest and negatively charged particle in all the atoms Properties of the eighth element similar to the first Sequential change in molecular formulaeĪverage of the first and the third atomic mass Properties of the eighth element similar to the firstį. Average of the first and the third atomic massĭ. Lightest and negatively charged particle in all the atomsĬ. Rearrange the columns 2 and 3 so as to match with the column 1. Periodic Classification Of Elements Question 1. Maharashtra State Board Class 10 Science Solutions Part 1 Chapter 2 Periodic Classification of Elements An interactive periodic table may be found here.Balbharti Maharashtra State Board Class 10 Science Solutions Part 1 Chapter 2 Periodic Classification of Elements Notes, Textbook Exercise Important Questions and Answers. \): The modern version of the periodic table.


 0 kommentar(er)
0 kommentar(er)
